Abstract
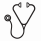
Background The EuroQuol 5 Dimension (EQ5D) is a validated tool to assess health-related quality of life (HRQoL). The International Prognostic Scoring System (IPSS) and the revised IPSS (R-IPSS) are the gold standards of prognostication in patients (pts) with myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML) and low blast count acute myeloid leukemia (AML). The EQ5D is the most used tool for patient reported outcome measures but is underused in MDS, CMML or AML, with only one report on the use of the updated 5 level (5L) published to date (Yu H, Qual Life Res 2021, 855).
Aims 1) Compare HRQoL of pts with MDS, CMML or AML with a German population norm (Grochtdreis T, Eur J Health Econ 2019, 933) matched by age, sex and no. of comorbidities. 2) Assess the impact of baseline HRQoL on azacitidine (AZA) treatment (trt) outcomes. 3) Longitudinal analyses of EQ-5D/lab value pairs per AZA cycle.
Methods Pts treated within the Austrian Azacitidine Registry were analyzed (NCT01595295, data cut-off 13 Dec 20). EQ5D and lab values were assessed at the start of AZA trt cycles. The EQ5D-5L German value set (Ludwig K, Pharmacoeconomics 2018, 663) and the reverse crosswalk tool provided by EuroQol were used for calculation of the EQ5D-index. Baseline characteristics with univariate p<0.10 (n=10) for association with OS were included in the multivariate (MV) regression. After stepwise selection, 6 variables remained and were used for MV adjustment (MVA). Outcome endpoints were overall survival (OS), time with clinical benefit (TCB) and time to next treatment (TTNT). Assign Data Management and Biostatistics GmbH performed statistical analyses with SAS® 9.4
Results 1456 EQ5D (n=225 3L; n=1231 5L) questionnaires were analyzed; 129, 33 and 110 of 272 pts had MDS, CMML or AML. Pts reported more pronounced restrictions in usual activities (+28%, p<·0001), anxiety/depression (+21%, <·0001), selfcare (+18%, p<·0001) and mobility (+15%, p<·0001), as well as lower mean EQ5D-5L indices (0·81 vs 0·88, p<·0001) and lower self-rated health on a visual analogue scale (VAS) (64 vs 72%, p<·0001) than a matched reference population.
Pts with an EQ5D available at AZA start (n=205) were stratified according to their Level Sum Score (LSS), EQ-VAS or EQ5D-index being </≥ the respective group median. After MVA, the EQ5D-index remained significant for OS (+5·0 mo; p=0·0143; HR=1·52 [1·09-2·13]), TCB (+3·0 mo; p=0·0258; HR=1·43 [1·04-1·95]) and TTNT (+3·0 mo; p=0·0332; HR=1·42 [1·03-1·96]) (Fig 1). Thus, an EQ5D-index <0·8845 at AZA start indicates an increased risk of death (+52%), shorter AZA trt duration (+43%) and a higher risk of requiring a next trt or dying (+42%) in MV adjusted analyses.
In univariate logistic regression, LSS (p·0009), EQ-VAS (p=0·0237) and EQ5D-index (p=0·0110) were correlated with response to AZA. After MVA, LSS remained significantly predictive of AZA response (p=0·0160; odds ratio (OR)=0·451 [0·235-0·852]), and the EQ5D-index showed a trend (p=0·0627; OR=0·522 [0·296-1·032]). A LSS of ≥8 at AZA start thus indicates a 0·45 times lower odds of responding to AZA.
Increases of the likelihood ratio (LR) were observed after addition of 1) LSS to the IPSS (p=0·0068) or R-IPSS (p=0·0205); 2) EQ-VAS to the IPSS (p=0·0031) or R-IPSS (p=0·0058); 3) EQ5D-index to the IPSS (p=0·0015) or R-IPSS (p=0·0012), indicating that they provide added value to the IPSS and the R-IPSS.
Up to 1432 "EQ5D response/clinical parameter" pairs revealed that hemoglobin level, platelet count, transfusion dependence, serum ferritin, creatinine, albumin, adverse events G3-4, AZA days and dose per cycle and hematologic improvement in erythrocytes and platelets significantly correlated with ≥5 of 8 EQ5D parameters.
Conclusions These data indicate for the first time that:
1) Patients with MDS, CMML or AML have profound impairments in HRQoL compared to a German population norm.
2) Baseline EQ5D-index predicts adjusted OS, TCB and TTNT.
3) Baseline LSS significantly predicts response to AZA.
4) Baseline LSS, EQ-VAS and EQ5D-index significantly add value to the existing gold standards for prognostication (IPSS, R-IPSS).
5) EQ5D responses during AZA trt correlated significantly with lab values associated with response, toxicity and AZA dose and no. of trt days per cycle.
Based on these results, we will continue to use the EQ5D-5L and recommend the longitudinal use of this brief questionnaire in both clinical trial- and real-world settings.
Pleyer: AbbVie, BMS, Novartis: Honoraria, Other: Travel support. Heibl: BMS: Honoraria. Leisch: Honoraria from BMS, Celgene, Gilead, Takeda and Novartis; Travel support: Celgene and Novartis: Honoraria, Other: Travel support. Melchardt: Abbvie, Celgene, Novartis: Honoraria. Wolf: Roche: Honoraria, Research Funding; MSD: Honoraria, Research Funding; BMS-Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria; Gilead: Honoraria; Incyte: Honoraria; GEMOAB: Honoraria. Greil: Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Sandoz: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Daiichi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Amgen: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Stauder: Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees.
While azacitidine is approved for all patients with MDS, CMML and AML by the US FDA, it is not approved for all patients subsets by EMA.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal